451 Seaview Parkway
Gulf View
La Romain
Trinidad
Trinidad & Tobago
868.652.4579/8334
Ext: 116/117
southernmed1987@gmail.com
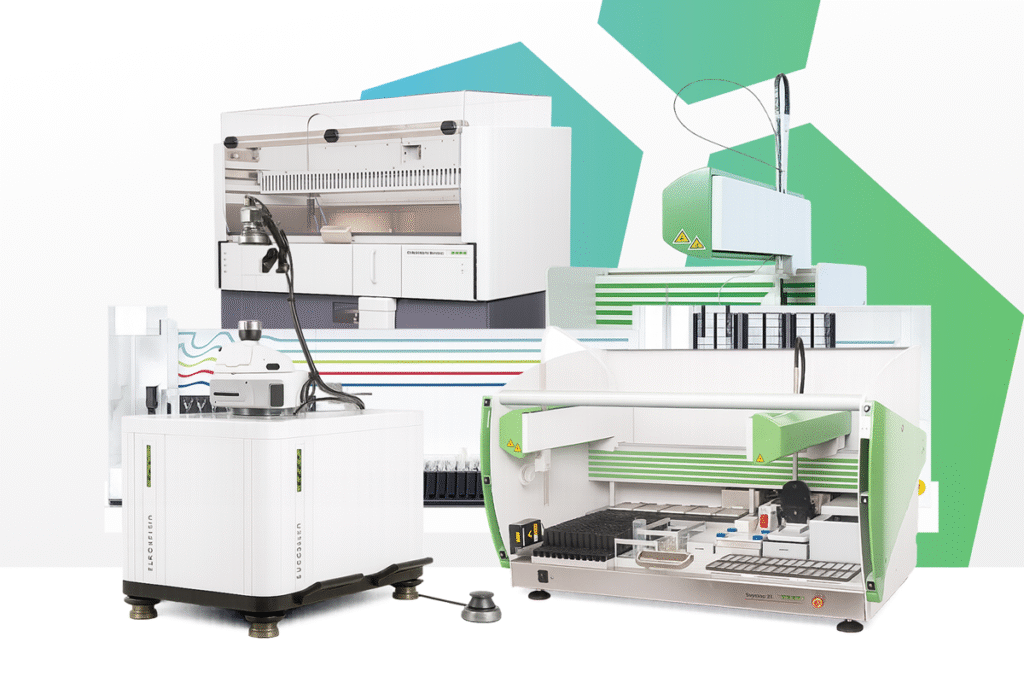
The EUROIMMUN EUROArray system represents a breakthrough in molecular diagnostics, combining the power of polymerase chain reaction (PCR) with advanced microarray technology to deliver rapid, reliable, and highly multiplexed genetic and infection testing. Designed with simplicity and standardisation at its core, EUROArrays allow laboratories to conduct complex molecular diagnostics in a streamlined, error-free workflow. In the Caribbean, these innovative solutions are available exclusively from Southern Medical Supplies Limited, your trusted partner for advanced molecular diagnostic technologies.
EUROArrays are uniquely designed for laboratories that need comprehensive yet efficient testing. Their multiplex capability allows the simultaneous detection of numerous DNA sequences in one test, making them ideal for molecular genetic diagnostics as well as molecular infection diagnostics. Each array is built on EUROIMMUN’s proprietary BIOCHIP technology and processed using TITERPLANE incubation, ensuring excellent standardisation and reliable hybridisation conditions.
To streamline laboratory workflows, all EUROArray PCR components are supplied ready-to-use, including DNA polymerase and validated primers. The simplified work protocols allow for fast, efficient, and error-free processing, significantly reducing the hands-on time required for molecular testing. Numerous integrated control reagents are embedded in every test to ensure the highest reliability of results, including quality checks for sample integrity, stringency of hybridisation, and absence of cross-contamination.
Fully automated and standardised evaluation and archiving is achieved through the EUROArrayScan software, which also enables LIS connectivity for secure bidirectional data transfer. Moreover, the EUROArray system includes innovative features such as EUROArray Direct, which eliminates the need for DNA isolation in many assays. With this approach, whole blood can be incubated directly with kit reagents and used immediately in PCR, further simplifying and accelerating the workflow.
The complete process, from sample preparation through to reporting, is validated according to the IVD Directive and CE-marked, giving laboratories complete confidence in the reliability and regulatory compliance of EUROArray testing.
The EUROArray platform integrates PCR amplification with DNA microarray hybridisation for precise detection of genetic markers and pathogens. Each EUROArray BIOCHIP contains 72 DNA spots, which allow duplicate determinations of up to 36 different DNA sequences, including internal controls. A single EUROArray slide contains five test fields, meaning that up to five patient samples can be analysed in parallel. For assays requiring broader panels, multiple BIOCHIPs can be coated onto a single field, increasing the number of detectable sequences and enabling large-scale multiplex testing.
During processing, DNA is either extracted using a conventional DNA isolation kit or prepared directly from whole blood when using the Direct procedure. Amplification is performed using ready-to-use EUROArray PCR reagents, and the target DNA sequences are labelled with fluorescent dyes during PCR. In the subsequent hybridisation step, the PCR products bind specifically to the complementary DNA probes immobilised on the EUROArray BIOCHIP. This incubation is performed under exact, standardised conditions using TITERPLANE technology, which guarantees reproducibility and consistency.
The finished arrays are read on the EUROArray Scanner, and results are analysed fully automatically by the EUROArrayScan software. Fluorescence signals are interpreted, documented, and archived digitally, with results reported directly into the LIS. This end-to-end process provides laboratories with a fast, reproducible, and secure solution for molecular diagnostics.
One of the unique advantages of EUROIMMUN EUROArrays is the availability of the Direct procedure, particularly for human genetic diagnostics. In this workflow, DNA isolation is not required. Instead, patient blood is incubated with extraction solutions provided in the kit, and the resulting sample can be used directly for PCR. This innovation reduces processing steps, eliminates potential error sources, and significantly accelerates time-to-result.
For all other parameters, DNA is retrieved using conventional isolation kits, after which the PCR and hybridisation proceed in the same streamlined fashion. In both workflows, laboratories benefit from ready-to-use PCR reagents and standardised protocols, ensuring accuracy and efficiency across all applications.
EUROIMMUN EUROArrays provide laboratories with the ability to perform highly multiplexed genetic and infectious disease testing in a single, simplified workflow. By combining BIOCHIP technology, TITERPLANE incubation, automated scanning, and fully digital evaluation, EUROArrays deliver a level of standardisation and reproducibility that few systems can match.
With options for direct use of blood samples, ready-to-use PCR reagents, and robust control systems, laboratories can rely on EUROArrays for accuracy, speed, and compliance with international standards. These systems make advanced molecular diagnostics accessible to laboratories of all sizes, empowering them to expand testing capacity while maintaining efficiency.
For laboratories in the Caribbean, the advantages of EUROIMMUN EUROArrays are fully supported by Southern Medical Supplies Limited, which provides complete access to the product range alongside training, service, and technical expertise. This ensures smooth integration and maximum benefit from the EUROArray system.
👉 Available now from Southern Medical Supplies Limited — your trusted partner for EUROIMMUN molecular diagnostics and EUROArray automation in the Caribbean.
With over three decades of regional expertise, SMSL is more than a supplier—we are your partner in healthcare innovation. By combining trusted global brands with unmatched local support, we deliver solutions that improve outcomes, reduce risks, and empower healthcare professionals to perform at their very best.






















